Background
During development and homeostasis, the human body relies on coordinated cell-to-cell communication across tissues and organs. In pathological states like cancer, tumor cells participate in this crosstalk, leading to selection pressures, clonal evolution, and tumor heterogeneity. Ultimately, tumors that emerge gain advantages that are either tumor-intrinsic or tumor-extrinsic. Tumor-intrinsic advantages, like sustained proliferation and immune evasion, directly influence cancer cell survival. Tumor-extrinsic advantages, such as immune suppression and angiogenesis, allow cancer cells to manipulate local or systemic crosstalk.
Immense progress has been made in mechanistic understanding of tumor-intrinsic factors that drive cancer progression; however, therapeutic targeting of these factors is often associated with therapy resistance and cancer recurrence. On the contrary, therapies that target tumor-extrinsic factors, such as immunotherapy, generate robust and durable responses in patients across various types of cancer and have emerged as the fourth pillar of cancer treatment. However, current immunotherapies are ineffective in approximately 50% of tumors, particularly immune-cold tumors. This underscores either the inefficacy of existing immunotherapeutic strategies or a lack of understanding regarding tumor-extrinsic factors in these types of tumors, or both. Addressing these knowledge gaps, I believe, can significantly enhance patient survival and reduce cancer mortality, and that is the grounding mission of my research program.
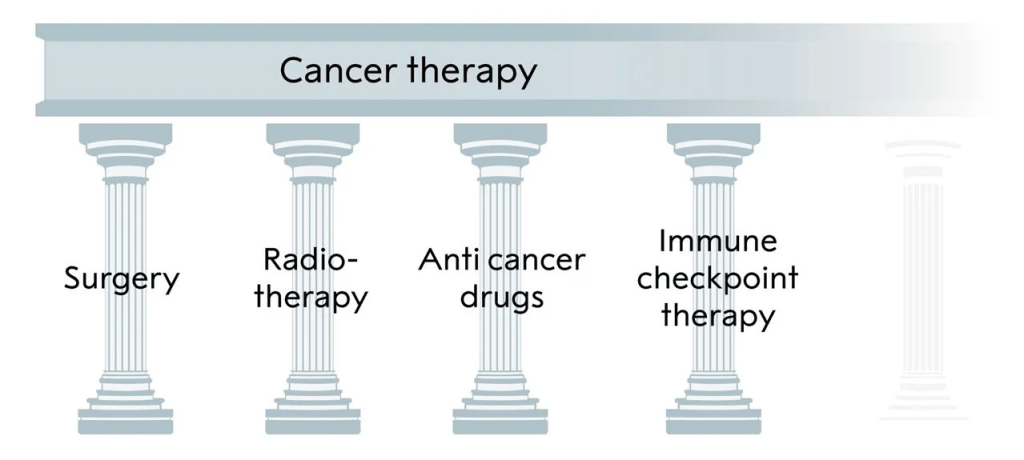
The three pillars of cancer treatment, all directed against the cancer cell, and the fourth, immune checkpoint inhibitor, based on unleashing an immune response against the tumor.
Major Research Projects
The Sohal Lab is actively working on the following research projects:
Extracellular vesicles mediated modulation of tumor biology
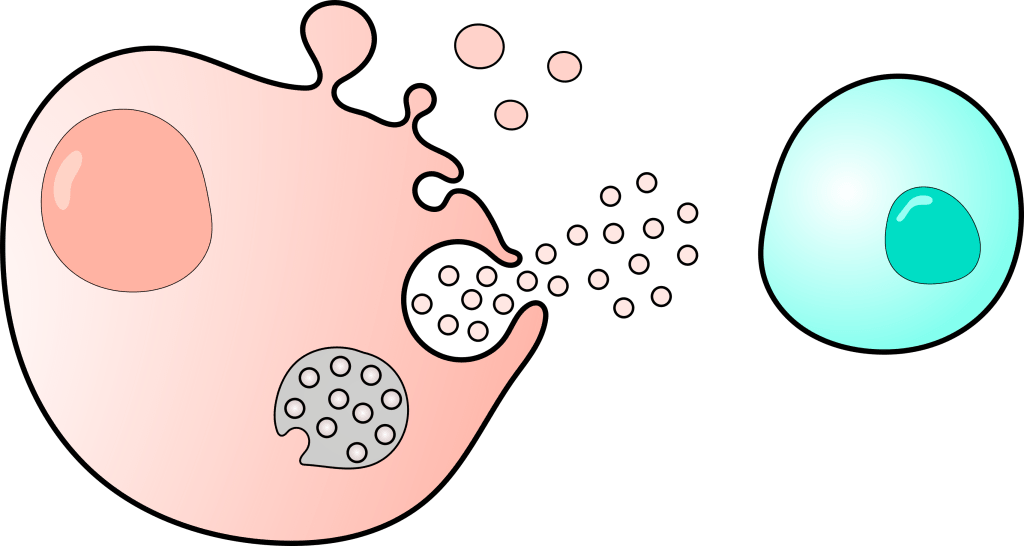
Extracellular vesicles (EVs) have undergone a paradigm shift from being considered as ‘waste bags’ to being central mediators of cell-to-cell communication in homeostasis and several pathologies including cancer. EVs have been shown to contribute to several cancer hallmarks – metabolic reprogramming, extracellular matrix remodeling, angiogenesis, immune evasion, and metastasis. However, these functions are attributed to the collective population of EVs released by a specific cell type or a tumor model, leaving a significant gap in understanding of the intricate EV-specific biology associated with cancer progression, such as the specific contributions of different EV subpopulations and EV biogenesis mechanisms. In this research area, we will dissect EV-mediated tumor biology and its specific role in contributing to different cancer hallmarks. Our ongoing work explores EV-mediated tumor-immune crosstalk and its role in immune evasion and resistance to immunotherapy.
RNA-based therapeutics targeting intercellular crosstalk in cancer
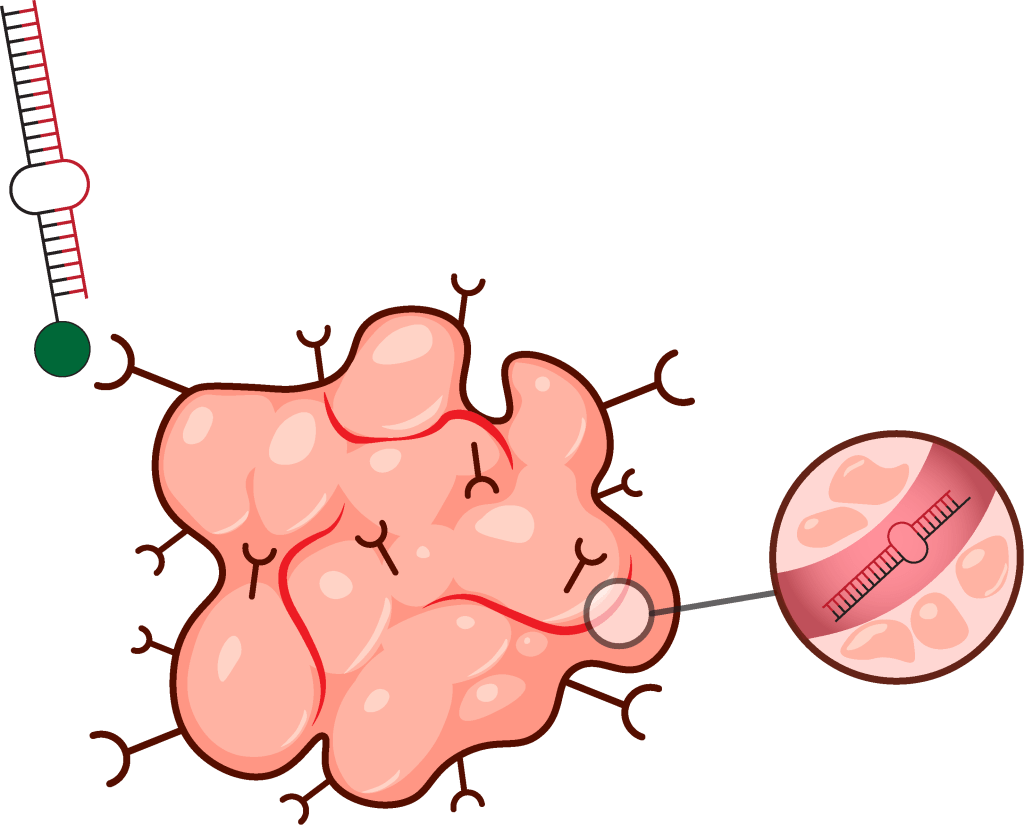
Historically, RNA-based therapeutics have faced delivery and stability challenges, including vehicle-associated toxicity and endosomal entrapment. Our prior experience in microRNA-based targeted cancer therapy has tackled these challenges in a significant manner. We designed a first-in-class chemically modified anti-tumor miRNA (miR-34a) with >400-fold increased stability, developed a ligand-mediated vehicle-free approach for targeted delivery of modified miR-34a to tumors in vivo, leading to reduced dosing yet sustained anti-tumor effect, and included additional moieties in the therapeutic design to promote endosomal escape. While mRNA-based immunotherapies are currently under investigation, utilization of microRNAs or siRNAs as immunotherapeutics remains underexplored. This research area will utilize synthetic RNA biology, patient-derived xenograft models, and clinical studies in collaboration with physician scientists to develop a novel class of miRNA/siRNA-based immunotherapies or enhance existing immunotherapies in immune-cold tumors.
Development of models to study novel intercellular crosstalk in cancer

How coding and non-coding genome modulates the intrinsic biology of a cell has been extensively studied, however, its role in modulating a cell’s crosstalk with other cell types is a frontier that remains significantly understudied. Here, by using physiologically-relevant in vitro and in vivo models, including humanized mice, leveraging cutting-edge genome modulation and single-cell omics approaches that preserve the spatiotemporal information of the intercellular crosstalk, cell and molecular biology, immunology, and extracellular vesicle biology, we aim to develop a comprehensive understanding of the genetic basis of cell-to-cell communication in cancer.
Please visit the Publications page to see our latest published work as well as work submitted to preprint servers. If interested in our research and/or potential collaboration, please contact Dr. Sohal at ikjot.sohal@unt.edu.
